What Is Ketamine
Ketamine is an unusual type of psychedelic substance — called a dissociative — that has surged in popularity. It reliably produces pain control, forgetfulness, disassociation, and euphoria, effects that underlie its medical and recreational uses.
Originally derived from PCP, or “angel dust,” ketamine has been used in hospitals and veterinary clinics as an anesthetic for decades. It’s also been cited as a psychedelic substance of misuse under the moniker “special K.”
More recently, Ketamine it has been widely used for treatment-resistant depression (TRD) — that is, severe depression that has not improved with several other therapies, including people who are experiencing suicidal thoughts.
Ketamine is the speedster of antidepressants, working within hours compared to more common antidepressants that can take several weeks.
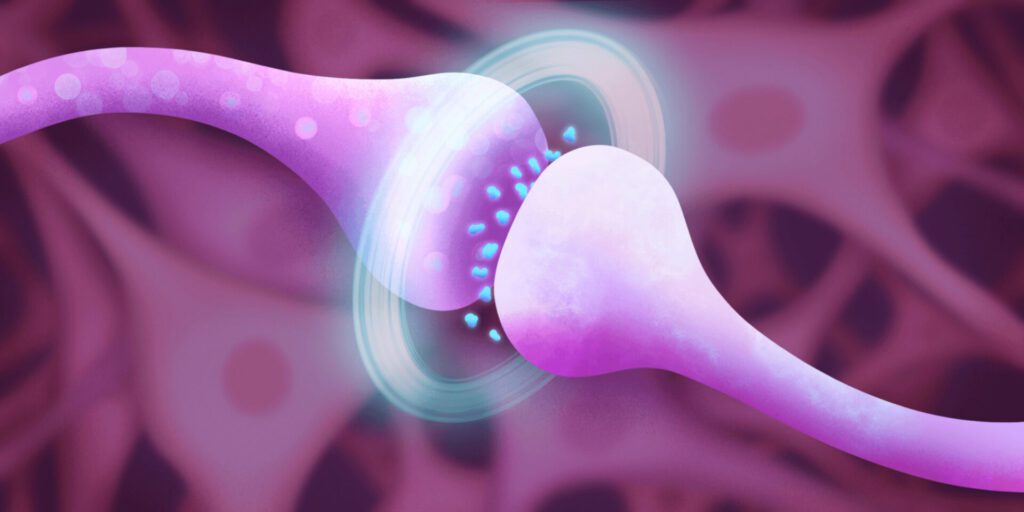
How do antidepressants work?
Research into ketamine as an antidepressant began in the 1990s , At the time (as is still mostly true today) depression was considered a “black box” disease, meaning that little was known about its cause.
One popular theory was the serotonin hypothesis, which asserted that people with depression had low levels of a neurotransmitter called serotonin. This hypothesis came about by accident—certain drugs given to treat other diseases like high blood pressure and tuberculosis seemed to drastically affect people’s moods. Those that lowered serotonin levels caused depression-like symptoms; others that raised serotonin levels created euphoric-like feelings in depressed patients. This discovery ushered in a new class of drugs meant to treat depression, known as selective serotonin reuptake inhibitors (SSRIs).
But eventually it became clear that the serotonin hypothesis didn’t fully explain depression. Not only were SSRIs of limited help to more than one-third of people given them for depression, but growing research showed that the neurotransmitters these drugs target (like serotonin) account for less than 20 percent of the neurotransmitters in a person’s brain. The other 80 percent are neurotransmitters called GABA and glutamate.
GABA and glutamate were known to play a role in seizure disorders and schizophrenia. Together, the two neurotransmitters form a complex push-and-pull response, sparking and stopping electrical activity in the brain. Researchers believe they may be responsible for regulating the majority of brain activity, including mood.
What’s more, intense stress can alter glutamate signaling in the brain and have effects on the neurons that make them less adaptable and less able to communicate with other neurons.
This means stress and depression themselves make it harder to deal with negative events, a cycle that can make matters even worse for people struggling with difficult life events.
Ketamine—from anesthetic to depression “miracle susbtance”

Interestingly, studies showed that the psychedelic substance Ketamine, which was widely used as anesthesia during surgeries, triggers glutamate production, which, in a complex, cascading series of events, prompts the brain to form new neural connections. This makes the brain more adaptable and able to create new pathways, and gives patients the opportunity to develop more positive thoughts and behaviors. This was an effect that had not been seen before, even with traditional antidepressants.
For the last two decades, researchers led Ketamine research by experimenting with using subanesthetic doses of Ketamine delivered intravenously in controlled clinic settings for patients with severe depression who have not improved with standard antidepressant treatments. The results have been dramatic: In several studies, more than half of participants show a significant decrease in depression symptoms after just 24 hours. These are patients who felt no meaningful improvement on other antidepressant medications.
Ketamine as an Antidepressant

Although it was initially developed as an anesthetic, Ketamine has exhibited other significant effects. For instance, during the last decade, it was recognized as an antidepressant. Consequently, it has recently been the focus of considerable research attention.
For instance, one study found that single-dose administration is associated with immediate antidepressant effects . Clinical studies have also shown that the low-dose administration of ketamine reduces the functional connectivity of the subgenual anterior cingulate cortex , a brain region that postmortem studies have indicated is significantly reduced in depressed patients . However, the functional effects are quite complex. One report found that the antidepressant effects of ketamine are associated with different types of alteration in brain oscillation. Specifically, theta and gamma oscillations were increased anteriorly, while theta, delta, and alpha oscillations were reduced posteriorly . These oscillations are functionally distinctive. For example, the theta wave is associated with learning, the alpha wave is linked to sleep cycles, the beta wave is associated with emotional and cognitive tasks, and the gamma wave is linked to the sensory and cognitive capacity . It is therefore important to determine the consequences of administering a subanesthetic dose of ketamine.
In addition, ketamine has been found to modulate glucose metabolisms in different brain regions associated with anxiety and depression , and is demonstrably effective in managing treatment-resistant depression .
Under physiological conditions, multiple mechanisms maintain energy supply to the neuronal populations. These dynamic mechanisms are affected by sleep, awakeness, and diseased states. Numerous psychiatric disorders have been reported to alter neurometabolic coupling, including depression. Based on postmortem tissue from depressed patients, proteomic study has reported >90 proteins involved in mechanisms regulating neurometabolic coupling. Implicating these homeostatic mechanisms in the pathology of depression . Furthermore, functional imaging studies have supported the involvement of brain energy metabolism in the pathology of depression.

Clinical settings have found that creatine phosphate and adenosine triphosphate levels were altered in multiple brain regions of depressed patients. In addition, the uptake of 18 (F)fluorodeoxyglucose, an indicator for glucose metabolism in neuronal populations, was found to be altered in multiple brain regions of depressed patients; these changes were reversed upon the administration of standard antidepressants. Of note, ketamine was found to reduce suicidal tendencies .
In the acute administration of ketamine, both synaptic connectivity and plasticity are potentiated. Preclinical studies have found that ketamine remedied the impairment in fear extinction memory in a model of depression . In support of this finding, d-cycloserine, another NMDA partial agonist, was shown to modulate the extinction of fear . The genetic mutations of GluN2B/NR2B, an NMDA receptor subunit, have been linked to brain disorders.
Final Thoughts
Ketamine has managed to reach molecular mechanisms that have not been reached by standard antidepressants. Clinical studies have also shown that the low-dose administration of ketamine reduces the functional connectivity of the subgenual anterior cingulate cortex , a brain region that postmortem studies have indicated is significantly reduced in depressed patients .